Solid / liquid / gas
+ some implications for Lab 4
We know of 3 common phases of matter:
- Solid
- Liquid
- Gas
Solid and liquid water in contact with each other
 ehe.osu.edu, beyond penguins
ehe.osu.edu, beyond penguins
Phases of matter
We know of 3 common phases of matter:
- Solid
- Liquid
- Gas (or Vapor)
Temperature
In your experience which of these phases tends to occur at the lowest / highest / in-between temperatures.
Remember that $$\frac32 k_BT=\frac12m\langle v^2 \rangle$$
What does this mean about the relative speeds in the 3 phases?
Atomic picture of the 3 phases
According to the atomic theory, these can be characterized as:
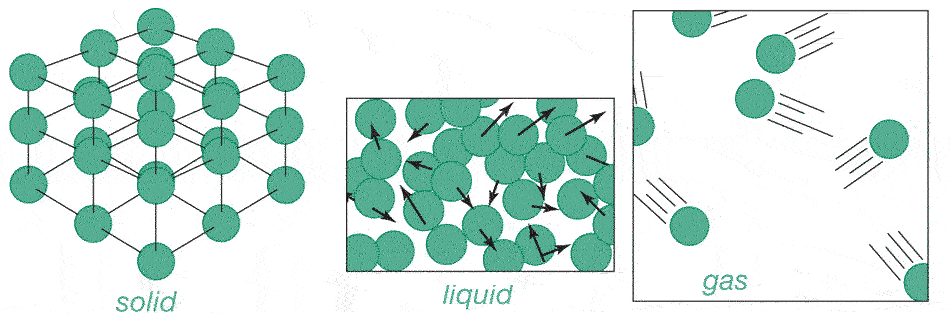
- Solid- The *average* position of any molecule is not changing.
(So, how can the atoms in a solid be moving if that's the case??)
- Liquid- The average position of a molecule is not constant. Molecules are
free to move past each other.
A liquid in a container (e.g. in a glass) will not fill the container.
- Gas- molecules are moving, the average density of a gas is much lower than a liquid. A gas will completely fill a container.
Go to this simulation: tiny.cc/statesofmatter. Pick "States" and pick "Argon".
- Find a temperature at which the material looks "solid".
- Can you tell if the "atoms" in this animation are attracted to each other or repelled from each other?
- Find a temperature at which the material looks to be a "gas".
- In between the solid and the gas there might be a "liquid" state. Can you find a temperature where that's the case?
- Was there ever a temperature at which more than one "state" is present?
- Any other observations?
Going from a liquid to a gas, atoms move somewhat faster, but also take up much more space. e.g. Water:
- 55.5 moles of liquid water takes up 1 liter of volume at 100 C.
- 55.5 moles of water vapor takes up 1300 liters at 100 C.
All atoms & molecules experience some mutual attraction! (There are no molecules that repel each other).
- Some molecules have a strong attraction. Thermal vibrations cannot easily break up the solid. They have high melting temperatures and high boiling points. Examples:
- Water ($H_2O$): Melting temperature: 0 C. Boiling temperature: 100 C.
- Antifreeze ($(CH_2OH)_2$): Melting temperature: -12.9 C. Boiling temperature: 197 C.
- Some molecules have a weak attraction to each other. Thermal vibrations can easily break up the solid. They have relatively low boiling points and low melting points. Examples:
- Carbon-dioxide ($CO_2$) goes straight to gas from solid $CO_2$ at -79 C.
- Nitrogen exists in our atmosphere as $N_2$. Melting temperature: -210 C: Boiling temperature: -196 C (77 K).
- Helium ($He$): Boiling temperature = -269 C (4.22 K)
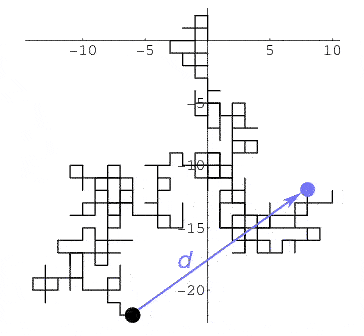
Einstein and Brownian motion
 Einstein
made some calculations (1905) about how grains buffeted by random molecular
collisions would move which agreed well with experiment.
Einstein
made some calculations (1905) about how grains buffeted by random molecular
collisions would move which agreed well with experiment.
Look up random walk or drunkard's walk. [children's activity]
- If a 'drunkard' wanders a city laid out on a square grid,
- at each intersection he randomly decides 1 of 4 possible directions to go next,
- he counts intersections as he goes, and counts $N$ of them.
- maybe passing through the same one many times--he doesn't know!
Einstein calculated that after he travels a total distance of $N$ intersections, he will on average be a distance $d=\sqrt{N}$ away from his starting point. [Though we have no idea of what direction away from his starting point he'll be.]
His calculation predicts that the distance a randomly buffeted particle moves away from where it started after a short time $\Delta t$, will be larger, the higher the temperature.
In 1926 Jean Baptiste Perrin won a Nobel prize for his work on sedimentation, and confirmation of Einstein's theory of Brownian motion.
[image: Wolfram math world]
Light and heat
Returning to our consideration of electro-magnetic waves. The frequency of a wave is related to how fast the hand is shaking.
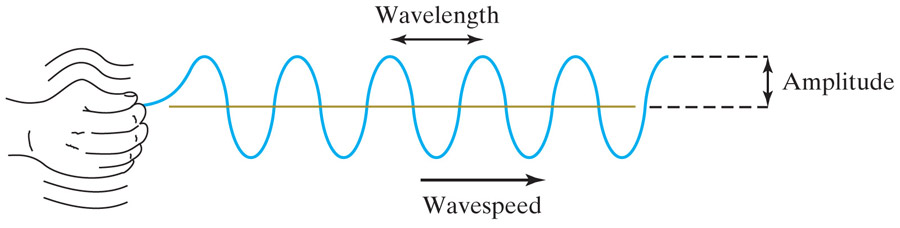
Can you guess what is playing the role of the hand? What is it that is doing the shaking that causes an E-M wave to be sent out?
What causes these wave to sometimes have a higher frequency (that we can see) or of a lower frequency (such as an IR wave)??
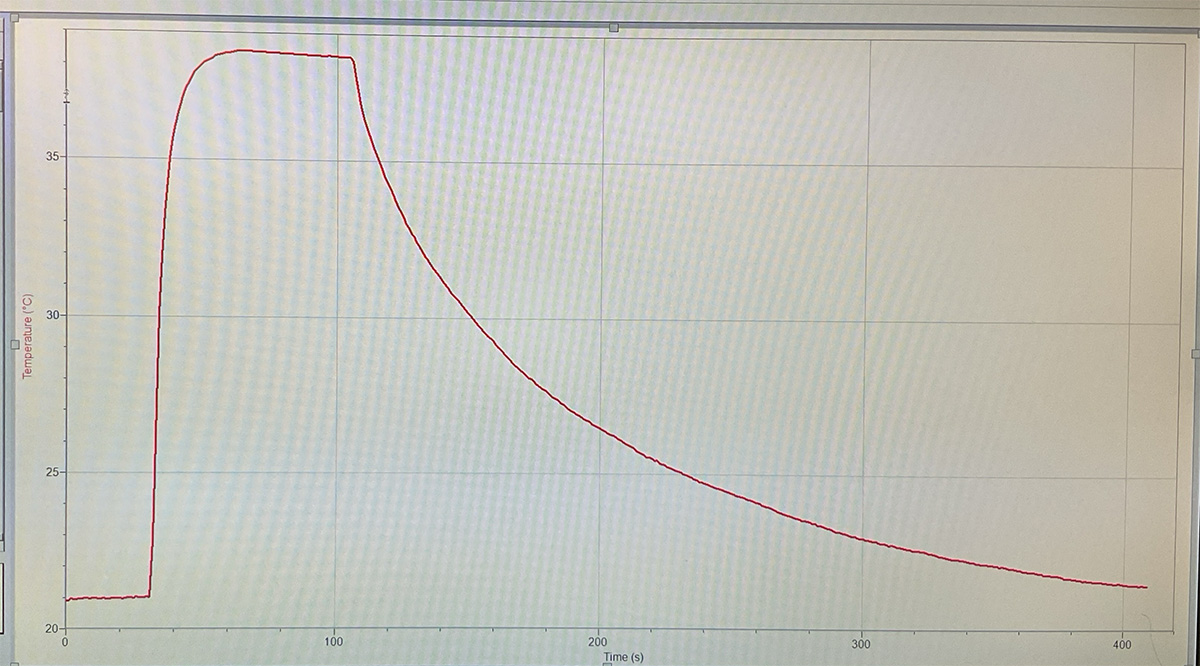
Lab 4 - thermal conductivity
What happens when a hot object (made of atoms) comes in contact with a cold object (made of atoms)?
Go to tiny.cc/chemheat and check out the Particle simulation of Thermal Conduction.
- one milliliter of water has a mass of 1 gram
- one milliliter of air has a mass of 0.00129 grams
How might that explain this?
Homework (hand it in on Moodle)
Read: Hobson, Chapter 2, Sections 1-3
Answer Concept Checks: 2, 4, 6, 7
Answer Conceptual exercises in Chapter 2: 3, 5, 7, 10, 16, 21, 22, 24
Image credits
Wikipedia - atmosphere, thermal motion